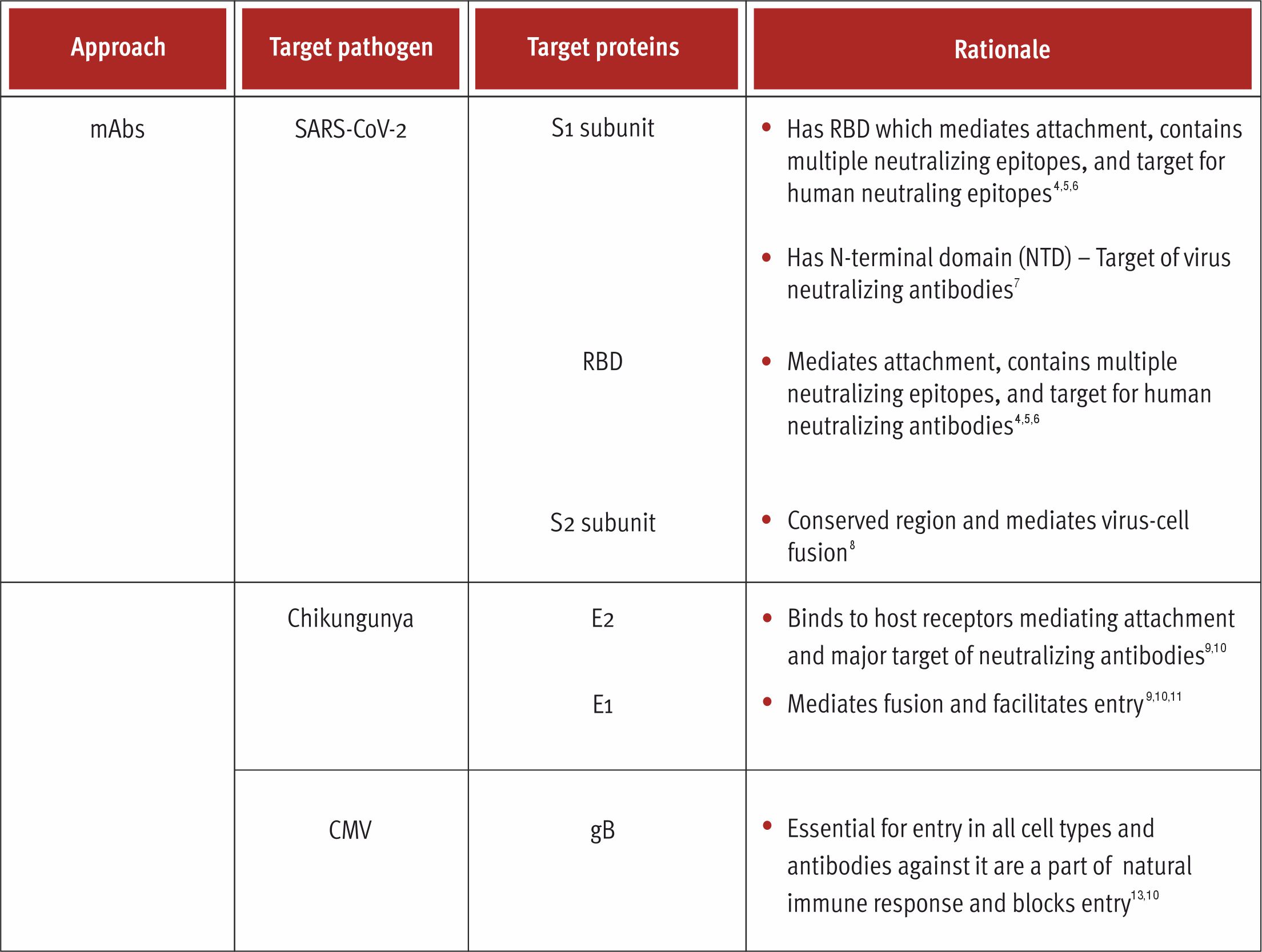
Monoclonal Antibodies (mAbs)
Immunization has its own limits with respect to the evolution of binding sites with ultra-high affinity, high stability and increased potency. Innovative strategies concerning antibodies enable the same, thus currently antibodies are critical detection reagents in research and diagnostics which have been developing rapidly in the biological therapeutics market. 1,4 Antibodies being one of the essential parts of the immune system have high affinity and are extremely specific while binding to different substances. It’s characteristics like long in vivo half-life and functions like complement-dependent cytotoxicity makes it a great tool for drug development.3
Additionally, they have useful properties for drug development such as long in vivo half-life and Fc-mediated functions such as complement-dependent cytotoxicity.
Antibodies are encoded by two genes, heavy chain and light chain, which can hinder the antibody library development. Phage display systems use fragments of antibodies since full-length functional antibodies cannot be produced sufficiently in the bacteria. 1
Reference:
- Hentrich, C., Ylera, F., Frisch, C., Ten Haaf, A., & Knappik, A. (2018). Monoclonal Antibody Generation by Phage Display: History, State-of-the-Art, and Future. In Handbook of Immunoassay Technologies (pp. 47-80). Academic Press.
- Hoogenboom, H. R. (2005). Selecting and screening recombinant antibody libraries. Nature
- Rajan, S., & Sidhu, S. S. (2012). Simplified synthetic antibody libraries. Methods in enzymology, 502, 3-23.
- Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165878. doi:10.1016/j.bbadis.2020.165878.
- Yu F, Xiang R, Deng X, et al. Receptor-binding domain-specifi human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2. Signal Transduct TargetTher. 2020;5{1):212. Published 2020 Sep 22. doi:10.1038/s41392-020-00318-o.
- Liu Z, Xu W, Xia S, et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct Target Ther. 2020;5{1):282. Published 2020 Nov 27. doi:10.1038/s41392-020-00402-5.
- Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev lmmunol. 2021;21(2):73-82. doi:10.1038/s41577-020-00480-o.
- HuangY, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141-1149. doi:10.1038/s41401-020-0485-4.
- Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127(3): 737-749. doi:10.1172/JCl84417.
- Singh A, Kumar A, Yadav R, Uversky VN, Giri R. Deciphering the dark proteome of Chikungunya virus. Sci Rep. 2018;8(1):5822. Published 2018 Apr 11. doi: 10.1038/s41598-018-23969-o.
- Kuo SC, Chen YJ, WangYM, et al. Cell-based analysis ofChikungunya virus E1 protein in membrane fusion. J Biomed Sci. 2012;19(1):44. Published 2012 Apr 21. doi:10.1186/1423-0127-19-44.
- Sandonfs V, Garcfa-Rfos E, McConnell MJ, Perez-Romero P. Role of Neutralizing Antibodies in CMV Infection: Implications for New Therapeutic Approaches. Trends Microbiol. 2020;28(11):900-912. doi:10.1016/j.tim.2020.04.003.
- McVoy MM, Tenorio E, Kauvar LM. A Native Human Monoclonal Antibody Targeting HCMV gB (AD-2 Site I). Int J Mal Sci. 2018;19(12):3982. Published 2018 Dec 11. doi:10.3390/ijms19123982.
- Mejias A, Rodrfguez-Fernandez R, Oliva S, Peeples ME, Ramilo 0. The journey to a respiratory syncytial virus vaccine. Ann Allergy Asthma lmmunol. 2020;125(1): 36-46. doi:10.1016/j.anai.2020.03.017.
